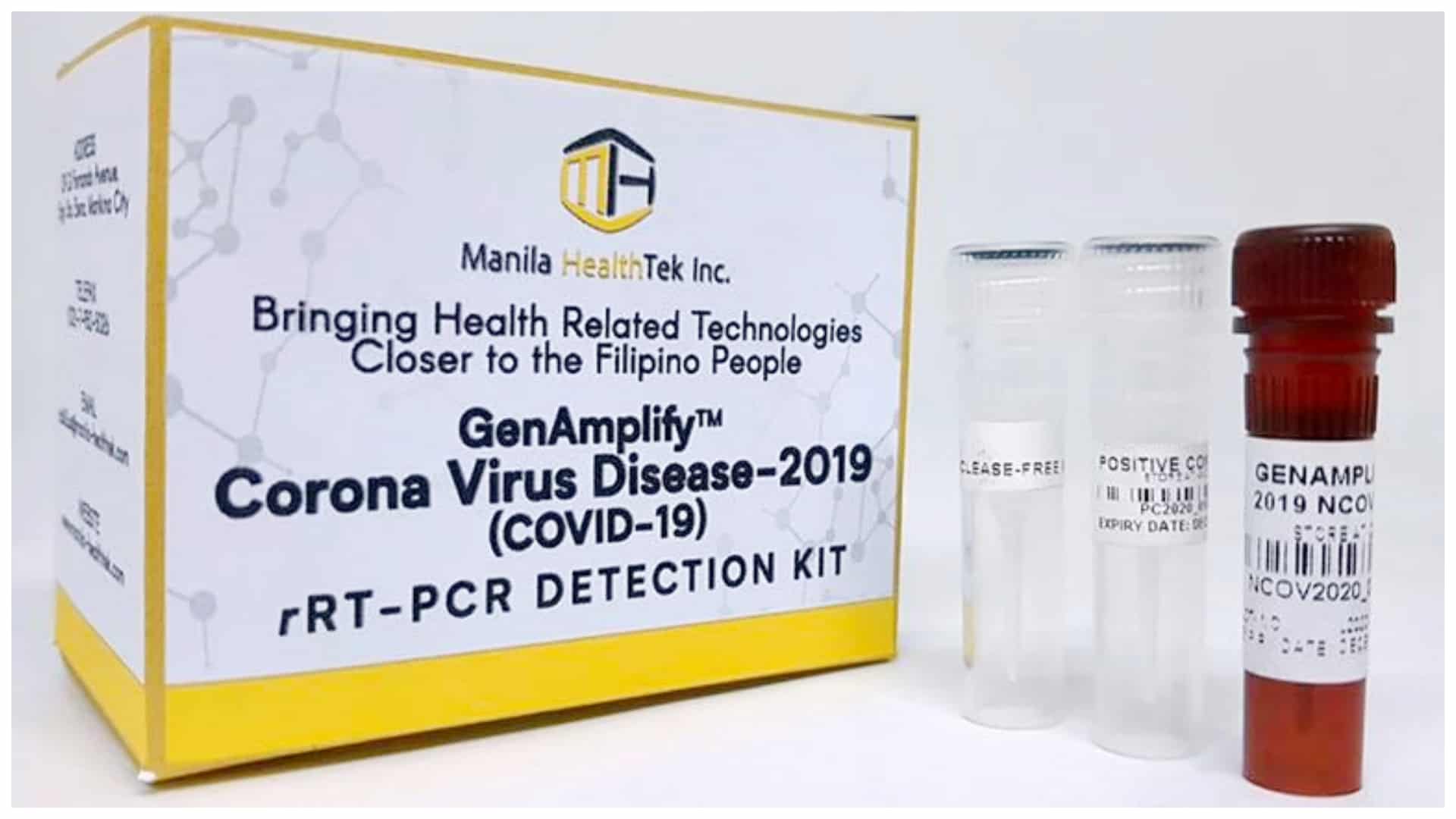
After five months since the outbreak began in the country, the Department of Health (DOH) has finally given the green signal for the use and mass production of these Filipino-made, quality yet less expensive, test kits.
“After several months of collaboration, we are proud to say, GenAmplify version 2, the country’s very own RT-PCR test kit, is finally ready for commercial use.”
– Department of Health (DOH)
It was last April the Food and Drug Administration (FDA) approved the commercial use of the locally made RT-PCR (real-time polymerase chain reaction) detection kit, funded by DOST and manufactured by Manila HealthTek, Inc. However, the test kits were recalled after a “very minor defect” was discovered the following month.
DOH has assured the Manila Health Tek team worked closely with their independent laboratory expert panel “in addressing key issues of version 1 of GenAmplify”.
Furthermore, DOH said that the same as the other medical products and devices, performance of the kit will be regularly monitored.
Compared to the foreign-made kits sold at P8,000, these locally-made kits will be sold at only P1,300 per piece.
“We, at the DOH and DOST-PCHRD, are proud of our homegrown scientists who continue to use their talent to benefit not only the Filipino people but the rest of humanity as well.”